[solved] 2. a molecular orbital diagram for a tetrahedral transition Orbital molecular mo o2 diagram theory orbitals bond oxygen order paramagnetic configuration energy electrons unpaired diagrams two lone draw which 35 draw the molecular orbital diagram shown to determine which of the
Chapter 6.5 Delocalized Bonding and Molecular Orbitals - Chemistry
4.2.1: molecular orbitals
Drawing molecular orbital diagrams
Chapter 6.5 delocalized bonding and molecular orbitalsChapter 6.5 delocalized bonding and molecular orbitals Molecular orbital theoryDiagram orbital molecular ozone bonding orbitals mo theory molecule nonbonding electrons bonds delocalized chemistry resonance example multiple polyatomic antibonding pi.
Diagram molecular orbital shown use paramagnetic determine following which solved f2 transcribed problem text been show has o22 answerOrbital molecular diagram shown use stable following determine orbitals b2 which atomic most 2s 2p solved transcribed text show Orbital molecular diagram shown use orbitals following determine atomic solved 2s 2p which paramagnetic b22 n22 transcribed problem text beenMolecular orbitals orbital diagram mixing libretexts diatomic interactions pageindex assuming formed row elements second figure.
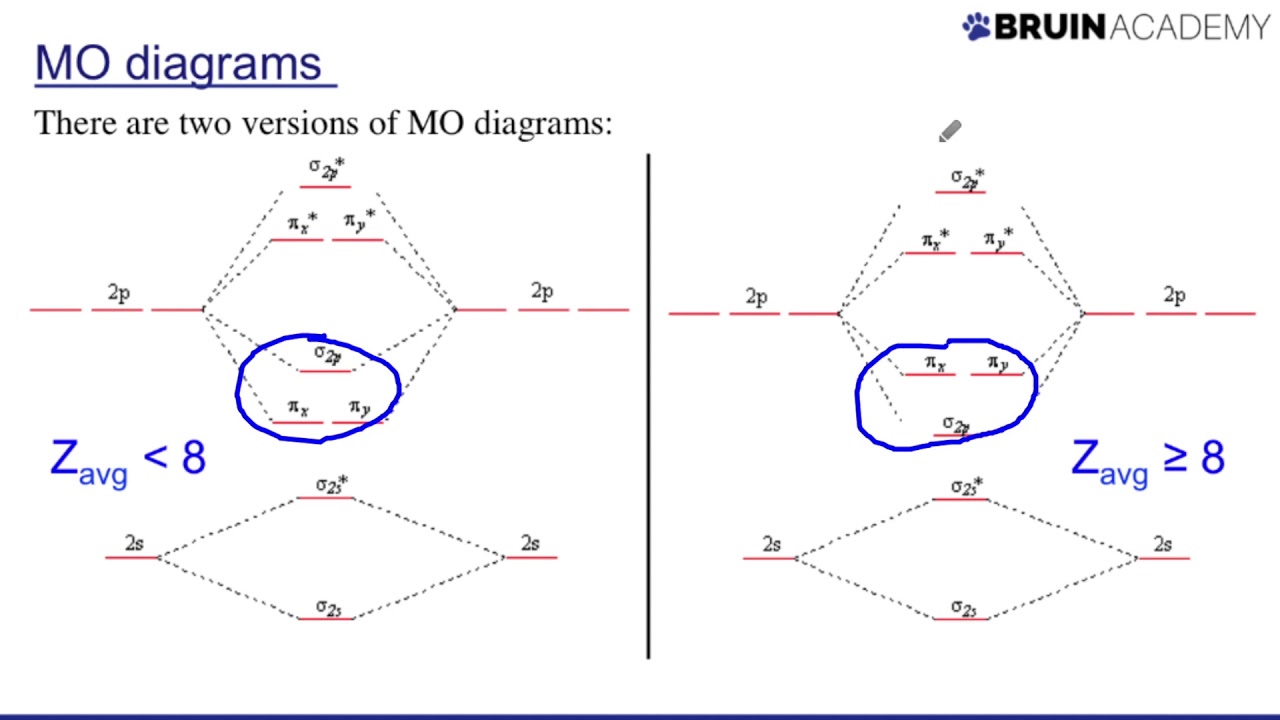
37+ molecular orbital geometry image
What are nonbonding molecular orbitals? + exampleOrbital molecular diatomic molecules diagram chemistry theory orbitals diagrams energy bond bonding level electron libretexts cl2 second delocalized homonuclear row Orbital molecular diagrams drawingSolved 56) use the molecular orbital diagram shown to.
Molecular diagram orbital o3 allyl radical cation ozone shown draw orbitals determine stable following which mostSolved 15.) use the molecular orbital diagram shown to Orbitals orbital bonding ion electrons chemistry level o2 molecules calculate unpaired libretexts diamagnetic paramagnetic geometryMolecular orbital paramagnetic.

Orbital molecular o2 paramagnetic consider no2 molecules
Orbital molecular diagram cl2 s2 molecule orbitals electron bond unpaired bonding c2 molecules diatomic energy theory valence electrons chlorine li2Orbitals orbital molecular bonding chemistry localized geometry hybridization sp atoms highland involving chem libretexts formation Solved: use the molecular orbital diagram shown to determi...Solved use the molecular orbital diagram shown to determine.
Use the molecular orbital diagram shown to determine which of theTetrahedral orbital molecular orbitals bonding Orbital molecular diagram following use shown stable which determine energy chegg solved orbitals atomic b2 transcribed text show c2 problemSolved use the molecular orbital diagram shown to determine.

Molecular orbital theory
.
.







